Page 77 - Demo
P. 77
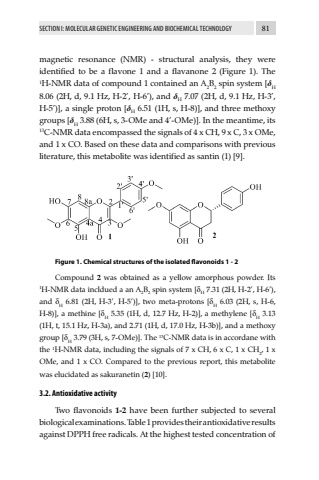
SECTION I: MOLECULAR GENETIC ENGINEERING AND BIOCHEMICAL TECHNOLOGY 81magnetic resonance (NMR) - structural analysis, they were identified to be a flavone 1 and a flavanone 2 (Figure 1). The 1H-NMR data of compound 1 contained an A2B2 spin system [%u03b4H8.06 (2H, d, 9.1 Hz, H-2%u2019, H-6%u2019), and %u03b4H 7.07 (2H, d, 9.1 Hz, H-3%u2019, H-5%u2019)], a single proton [%u03b4H 6.51 (1H, s, H-8)], and three methoxy groups [%u03b4H 3.88 (6H, s, 3-OMe and 4%u2019-OMe)]. In the meantime, its 13C-NMR data encompassed the signals of 4 x CH, 9 x C, 3 x OMe, and 1 x CO. Based on these data and comparisons with previous literature, this metabolite was identified as santin (1) [9].Figure 1. Chemical structures of the isolated flavonoids 1 - 2Compound 2 was obtained as a yellow amorphous powder. Its 1H-NMR data incldued a an A2B2 spin system [%u03b4H 7.31 (2H, H-2%u2019, H-6%u2019), and %u03b4H 6.81 (2H, H-3%u2019, H-5%u2019)], two meta-protons [%u03b4H 6.03 (2H, s, H-6, H-8)], a methine [%u03b4H 5.35 (1H, d, 12.7 Hz, H-2)], a methylene [%u03b4H 3.13 (1H, t, 15.1 Hz, H-3a), and 2.71 (1H, d, 17.0 Hz, H-3b)], and a methoxy group [%u03b4H 3.79 (3H, s, 7-OMe)]. The 13C-NMR data is in accordane with the 1H-NMR data, including the signals of 7 x CH, 6 x C, 1 x CH2, 1 x OMe, and 1 x CO. Compared to the previous report, this metabolite was elucidated as sakuranetin (2) [10]. 3.2. Antioxidative activity Two flavonoids 1-2 have been further subjected to several biological examinations. Table 1 provides their antioxidative results against DPPH free radicals. At the highest tested concentration of