Page 64 - Demo
P. 64
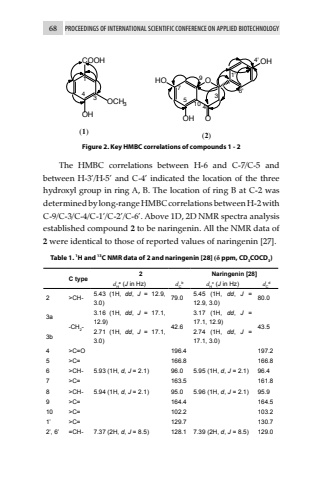
68 PROCEEDINGS OF INTERNATIONAL SCIENTIFIC CONFERENCE ON APPLIED BIOTECHNOLOGYCOOHOHOCH313 4 (1)47HO 9 OOHOH O31'4'6'5 10 (2)Figure 2. Key HMBC correlations of compounds 1 - 2The HMBC correlations between H-6 and C-7/C-5 and between H-3%u2019/H-5%u2019 and C-4%u2019 indicated the location of the three hydroxyl group in ring A, B. The location of ring B at C-2 was determined by long-range HMBC correlations between H-2 with C-9/C-3/C-4/C-1%u2019/C-2%u2019/C-6%u2019. Above 1D, 2D NMR spectra analysis established compound 2 to be naringenin. All the NMR data of 2 were identical to those of reported values of naringenin [27].Table 1. 1H and 13C NMR data of 2 and naringenin [28] (%u03b4 ppm, CD3COCD3)C type2 Naringenin [28]dHa (J in Hz) dCb dHc (J in Hz) dCd2 >CH- 5.43 (1H, dd, J = 12.9, 3.0) 79.0 5.45 (1H, dd, J = 12.9, 3.0) 80.03a-CH2-3.16 (1H, dd, J = 17.1, 12.9) 42.63.17 (1H, dd, J = 17.1, 12.9) 43.53b 2.71 (1H, dd, J = 17.1, 3.0)2.74 (1H, dd, J = 17.1, 3.0)4 >C=O 196.4 197.25 >C= 166.8 166.86 >CH- 5.93 (1H, d, J = 2.1) 96.0 5.95 (1H, d, J = 2.1) 96.47 >C= 163.5 161.88 >CH- 5.94 (1H, d, J = 2.1) 95.0 5.96 (1H, d, J = 2.1) 95.99 >C= 164.4 164.510 >C= 102.2 103.21%u2019 >C= 129.7 130.72%u2019, 6%u2019 =CH- 7.37 (2H, d, J = 8.5) 128.1 7.39 (2H, d, J = 8.5) 129.0